Abstract
Purpose: The aim of this study was to determine whether the efficacy and tolerability of subcutaneous cytarabine injection are non inferior to those of continuous intravenous infusion of cytarabine for patients with de novo acute myeloid leukemia.
Methods: Patients with de novo acute myeloid leukemia were randomized into two groups: Patients in group A received idarubicin 10 mg/m2 for 3 days and subcutaneous cytarabine 50 mg/m2 injection twice daily for 7 days. Patients in group B received idarubicin 10 mg/m2 for 3 days and intravenous cytarabine 100 mg/m2 by continuous infusion daily for 7 days. The primary efficacy end-point was complete remission. A lower confidence bound greater than -15% indicated noninferiority.
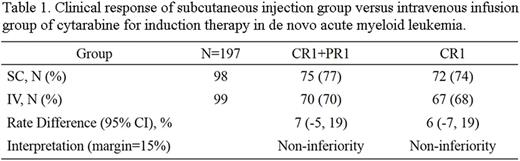
Results: One hundred and ninety-eight patients were analyzed in this study. The demographic characteristics were generally similar between two groups. The first complete remission rate was 74% and 68% for subcutaneous injection and intravenous infusion cytarabine groups, respectively. The difference between these two groups was 6%, with confidence interval ranging from 7% to 19%. These results indicated subcutaneous cytarabine injection group was non inferior to continuous intravenous infusion group with regard to the primary end point (Table 1). At the same time, adverse events were comparable between two groups.
Conclusion: The efficacy and tolerability of cytarabine subcutaneous injection was found to have been non inferior to those of intravenously continuous infusion cytarabine for the standard induction therapy. Cytarabine subcutaneous injection offers a convenient alternative to therapy acute myeloid leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal